Overview
The research in our lab stems from a fascination with the functional diversity of metabolites 1. Although metabolites are central to all biological processes, we often refer to metabolomes as dark matter, as we do not understand the full chemical complexity of even well-studied model organisms. But dark matter is equally suited to describe the poor understanding of roles and modes of action of many known compounds. Our work aims to address this gap by exploiting recent advances in mass spectrometry (MS) and the diverse biochemical methods for proteome and metabolome-wide characterization of protein-metabolite complexes 2,3. The overarching goal is to identify and functionally characterize protein-metabolite interactions (PMIs), prioritizing metabolite-protein pairings that are evolutionarily conserved and associated with processes essential to organismal health and resilience, most prominently, but not exclusively, central metabolism. The basic knowledge accrued through our work addresses a fundamental biological question: how organisms exploit small molecules to regulate cellular functions. The outcome of my research may lead to innovation in the form of small molecule-based strategies aimed at improving plant and animal health.
Don’t let go – co-fractionation mass-spectrometry (CF-MS) to map specific and pan-organismal protein-metabolite interactomes
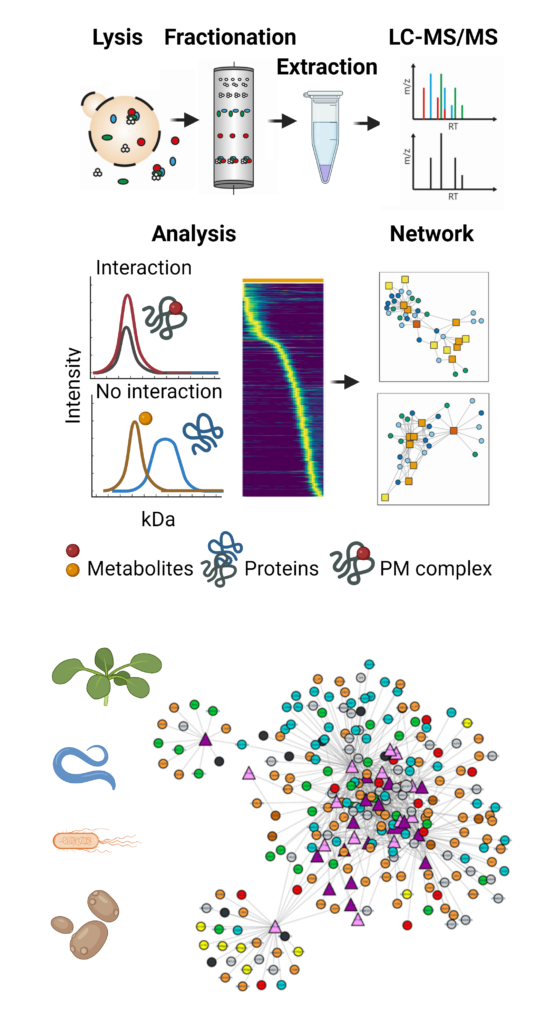
The lab developed a CF-MS-based strategy to provide a snapshot of the protein-protein and protein-metabolite interactome in a single experiment. We named our method PROMIS, PROtein-Metabolite Interactions using Size separation 4–7. PROMIS relies on size-based separation of molecular complexes in native cell lysate. In contrast to free small molecules, metabolites bound to protein complexes will separate into earlier-eluting protein-containing fractions. Chromatographic fractions are subjected to untargeted MS-based proteomic and metabolomic analysis. The similarity of the resulting protein and metabolite elution profiles is compared; we use a user-friendly, web-based app called PROMIsed 8. Co-fractionation is used to determine putative interactors. Recently, to improve the accuracy of our analysis, we have integrated PROMIS with an orthogonal ion exchange separation 9. The output of our work is protein-metabolite interaction networks. An advantage of CF-MS-based approaches 10, such as PROMIS, to assess PMIs is that they offer a direct and largely unbiased view into the interactome and retrieve interactions that can’t be inferred based on traditional metabolomic or proteomic analysis. This includes interactions between proteins and metabolites, whose chemical structure remains to be elucidated. In the last five years, the group has generated multiple CF-MS datasets in E. coli, budding yeast S. cerevisiae, plant A. thaliana, and roundworm C. elegans. In addition to assembling organism-specific interactomes, we plan to use these to map the pan-organismal PMI network to select evolutionarily conserved protein-metabolite pairings with functions in plant and animal health. Please contact us if you would like to learn more about using CF-MS to map protein-metabolite interactions in your favorite system.
Starting with the metabolite or protein bait
To complement PROMIS, the group uses targeted methods that start with either protein or metabolite bait to fish out interactors. When starting with a protein, we adapted a tandem affinity purification 11,12 protocol where the protein of interest is fused to an affinity tag. When starting with a metabolite, we have experience with affinity purification, where the small molecule of interest is coupled to the agarose beads 13, and with more recent techniques, such as thermal proteome profiling 14, which examines the change in protein thermal stability upon ligand binding.
Small but mighty, proteinogenic dipeptides acting at the nexus of protein degradation and metabolism.
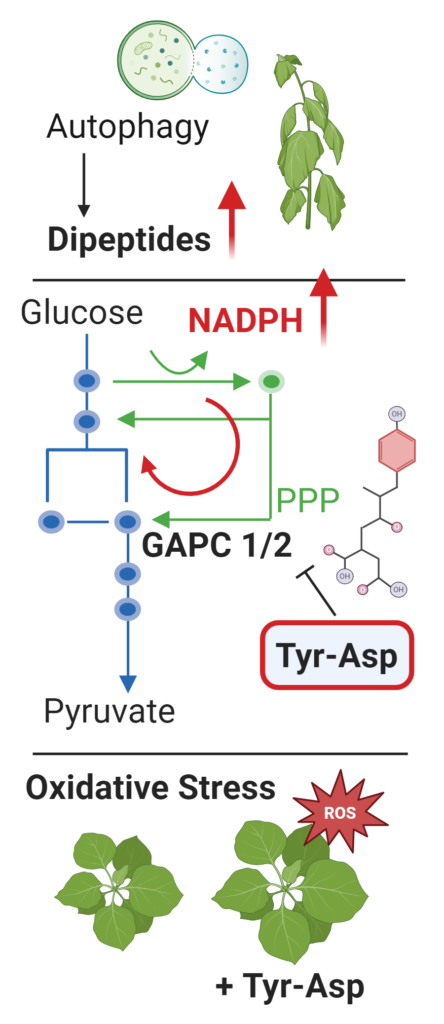
Proteogenic dipeptides constitute a large and chemically diverse group of compounds found in protein complexes 15. Dipeptides originate through protein degradation, and although they are always present in the cell, their levels strongly respond to the environment, often in a dipeptide-specific fashion 16,17. Exogenously applied dipeptides have many health-promoting activities, but we know little about their exact modes of action. Our analysis across plants, yeast, and E. coli identified an extensive protein-dipeptide interaction network enriched in central metabolism enzymes 4,6,9. By focusing on selected dipeptide-enzyme pairings in yeast and plants, we demonstrated that dipeptides regulate enzymatic activities affecting metabolic flux distribution. For instance, binding of a dipeptide Tyr-Asp to the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH/GAPC) inhibits its activity, redirecting glycolytic flux to the pentose phosphate pathway and NADPH production, and boosts plant resistance towards oxidative stress 18. These data are supported by the observed autophagy-dependent accumulation of aspartic and glutamic acid-containing dipeptides in response to abiotic stress, such as heat, which we also showed is autophagy-dependent 17. We continue pursuing the role of specific dipeptides in regulating distinct points of flux control and dipeptide contribution to metabolic reprogramming in response to environmental changes.
Metabolites involved in plant-microbe interactions
Metabolites play an important role in plant-microbe interactions. Pseudomonas syringae is a common bacterial plant pathogen and an established model for studying plant-pathogen interactions. To better understand the roles of plant and microbial-derived metabolites in virulence and immunity, we mapped the protein-metabolite interactome of Arabidopsis leaves infected with Pseudomonas syringae. The resulting network comprises tens of novel interactions between plant and bacterial compounds and plant proteins. We are currently pursuing selected pairings and their role in plant immunity.
The Secret(ed) Life of Aphid Salivary Metabolites in the Plant
Plant-aphid interactions are a complicated relationship that tells the story of the arm’s race for resistance. Aphids weave through the epidermal and mesophyll cells of the plant to tap into the phloem and feed for long periods (8-24 hours) on a single phloem cell where they encounter a myriad of plant defenses. To counteract these defenses, and aid in digesting plant material, aphids will spit a complex mixture of proteins and small molecules into the plant. While aphid salivary proteins are well characterized, the role of salivary metabolites remains to be unearthed. The goal of this project is to characterize the composition of metabolites in multiple aphid species and discover their role in altering plant metabolism and/or the resistance response. In collaboration with Dr. Georg Jander at the Boyce Thompson Institute, Ithaca, US.
2’, 3’- and 3’, 5’ – cyclic adenosine monophosphate; so similar yet so different
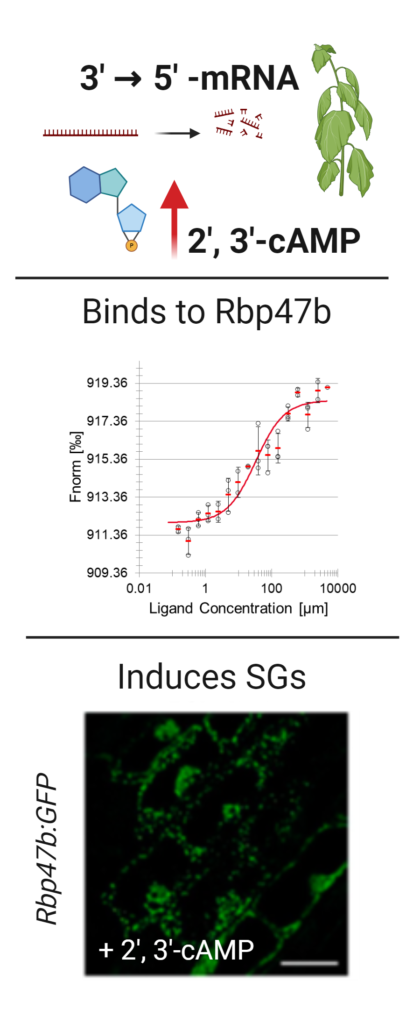
Building on the PROMIS-derived PMI network, we demonstrated that 2’, 3’ -cAMP, a poorly characterized RNA degradation product triggers stress responses 19 and binds to the RNA binding motif of Rbp47b protein 13, facilitating stress granules (SGs) assembly. SGs are evolutionarily conserved aggregates of proteins and RNAs that form in response to stress. Once sequestered within SGs, proteins, and RNAs are protected from harmful conditions. As a follow-up to the 2’, 3’-cAMP work, we adapted the protocol for SGs isolation and reported the first proteome composition of SGs in plants 20. Significantly, we demonstrated that SGs, in addition to RNAs and proteins, sequester metabolites and that SGs-like foci form in the plant plastids 21. Our findings are significant as they reveal the existence of a regulatory loop linking RNA degradation with RNA protection and a novel functionality of SGs, that is, metabolite sequestration. In comparison, a positional isomer of 2’, 3’-cAMP, 3’, 5’-cAMP is a well-characterized signaling molecule. By mapping 3’, 5’-cAMP interactome in Arabidopsis, we could demonstrate its role in regulating the actin cytoskeleton and, consequently, plant growth 14. This work is now continued by the lab alumni, Dr. Monika Chodasiewicz.
Publications
- Kosmacz, M., Sokołowska, E. M., Bouzaa, S. & Skirycz, A. Towards a functional understanding of the plant metabolome. Current Opinion in Plant Biology vol. 55 47–51 (2020).
- Wagner, M., Zhang, B., Tauffenberger, A., Schroeder, F. C. & Skirycz, A. Experimental methods for dissecting the terra-incognita of protein-metabolite interactomes. Current Opinion in Systems Biology 100403 (2021).
- Luzarowski, M. & Skirycz, A. Emerging strategies for the identification of protein–metabolite interactions. J. Exp. Bot. 70, 4605–4618 (2019).
- Veyel, D. et al. PROMIS, global analysis of PROtein–metabolite interactions using size separation in Arabidopsis thaliana. J. Biol. Chem. 293, 12440–12453 (2018).
- Veyel, D. et al. System-wide detection of protein-small molecule complexes suggests extensive metabolite regulation in plants. Sci. Rep. 7, 42387 (2017).
- Luzarowski, M. et al. Global mapping of protein–metabolite interactions in Saccharomyces cerevisiae reveals that Ser-Leu dipeptide regulates phosphoglycerate kinase activity. Communications Biology 4, 181 (2021).
- Schlossarek, D. et al. Rewiring of the protein-protein-metabolite interactome during the diauxic shift in yeast. Cell. Mol. Life Sci. 79, 550 (2022).
- Schlossarek, D. et al. PROMISed: A novel web-based tool to facilitate analysis and visualization of the molecular interaction networks from co-fractionation mass spectrometry (CF-MS) experiments. Comput. Struct. Biotechnol. J. 19, 5117–5125 (2021).
- Wagner, M. et al. Mapping protein-metabolite interactions inE. coliby integrating chromatographic techniques and co-fractionation mass spectrometry. bioRxiv (2024) doi:10.1101/2024.02.14.580258.
- Schlossarek, D. et al. Don’t let go: co-fractionation mass spectrometry for untargeted mapping of protein-metabolite interactomes. Plant J. 113, 904–914 (2023).
- Luzarowski, M. et al. Affinity purification with metabolomic and proteomic analysis unravels diverse roles of nucleoside diphosphate kinases. J. Exp. Bot. 68, 3487–3499 (2017).
- Wojciechowska, I. et al. Arabidopsis PROTODERMAL FACTOR2 binds lysophosphatidylcholines and transcriptionally regulates phospholipid metabolism. New Phytologist (2024).
- Kosmacz, M. et al. Interaction of 2′,3′-cAMP with Rbp47b Plays a Role in Stress Granule Formation. Plant Physiol. 177, 411–421 (2018).
- Figueroa, N. E. et al. Protein interactome of 3’,5′-cAMP reveals its role in regulating the actin cytoskeleton. Plant J. 115, 1214–1230 (2023).
- Minen, R. I., Thirumalaikumar, V. P. & Skirycz, A. Proteinogenic dipeptides, an emerging class of small-molecule regulators. Curr. Opin. Plant Biol. 75, 102395 (2023).
- Calderan-Rodrigues, M. J. et al. Proteogenic Dipeptides Are Characterized by Diel Fluctuations and Target of Rapamycin Complex-Signaling Dependency in the Model Plant Arabidopsis thaliana. Front. Plant Sci. 12, 758933 (2021).
- Thirumalaikumar, V. P., Wagner, M., Balazadeh, S. & Skirycz, A. Autophagy is responsible for the accumulation of proteogenic dipeptides in response to heat stress in Arabidopsis thaliana. FEBS J. (2020) doi:10.1111/febs.15336.
- Moreno, J. C. et al. Tyr-Asp inhibition of glyceraldehyde 3-phosphate dehydrogenase affects plant redox metabolism. EMBO J. 40, e106800 (2021).
- Chodasiewicz, M. et al. 2’,3′-cAMP treatment mimics the stress molecular response in Arabidopsis thaliana. Plant Physiol. (2022) doi:10.1093/plphys/kiac013.
- Kosmacz, M. et al. Protein and metabolite composition of Arabidopsis stress granules. New Phytol. 222, 1420–1433 (2019).
- Chodasiewicz, M. et al. Identification and Characterization of the Heat-Induced Plastidial Stress Granules Reveal New Insight Into Arabidopsis Stress Response. Front. Plant Sci. 11, 595792 (2020).
